UAMS Receives FDA Authorization for Convalescent Plasma Use, Plus Partnerships for Statewide Access
| The Food and Drug Administration has authorized the use of convalescent plasma in Arkansas as an experimental treatment for COVID-19 with the University of Arkansas for Medical Sciences (UAMS) as the expanded access treatment program sponsor.
With the authorization, UAMS Medical Center is able to use the treatment now without applying for FDA approval for every patient. Other health care providers in the state can also work through the UAMS Arkansas Expanded Access COVID-19 Convalescent Plasma Treatment Program to use the treatment.
The effort is a partnership between UAMS, the Arkansas Department of Health, UAMS Transfusion Medicine Services (Blood Bank), UAMS Office of Research Regulatory Affairs in the Division of Research & Innovation, UAMS Translational Research Institute, blood collectors such as the Arkansas Blood Institute, and the FDA.
“This ensures that convalescent plasma collected in Arkansas will benefit Arkansas patients,” said Tina S. Ipe, M.D., M.P.H., associate professor and division director for UAMS Transfusion Medicine Services and project leader for the statewide convalescent plasma effort. “If we had simply participated in an established nationwide program, much of what was donated here would have gone to hotspots elsewhere. The creation of this program gives us the chance to explore this potential treatment in Arkansas, for Arkansans.”
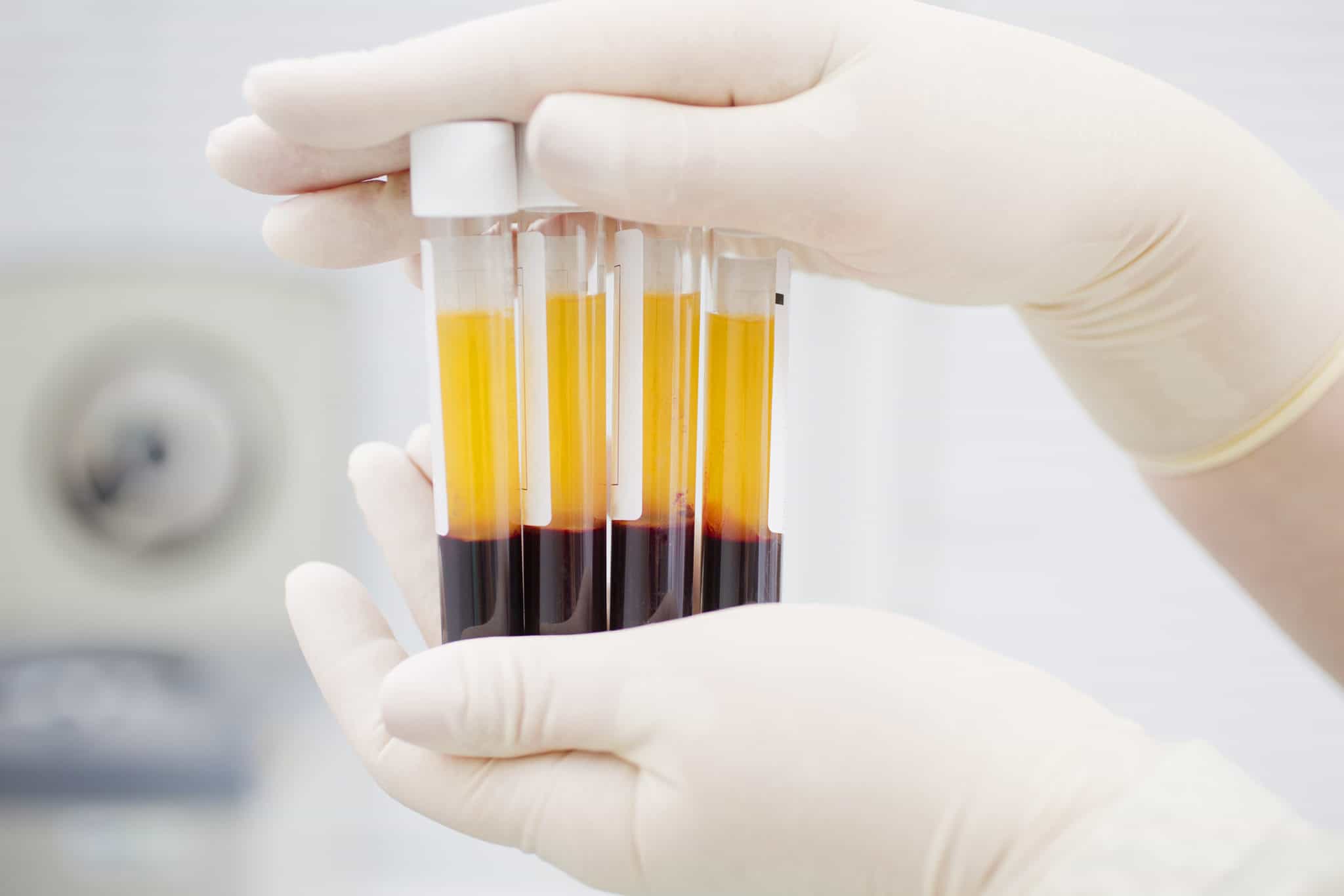
Covid convalescent plasma (CCP) is the liquid part of blood that is collected from individuals who have recovered from COVID-19, the illness caused by the new coronavirus. The plasma contains antibodies, which for some viruses, can help fight infection. Because there is no FDA-approved therapy for this disease presently, scientists across the world are testing COVID-19 convalescent plasma’s potential use as a treatment for people with COVID-19. The antiviral drug remdesivir has also shown promise, and the FDA has also authorized its investigational use.
“This program is only possible because of the hard work of many people who were able to get this approval in record time,” said Shuk-Mei Ho, Ph.D., as UAMS vice chancellor for research and innovation. “Creating partnerships, writing protocols and getting FDA and IRB (Institutional Review Board) approval can usually take several months to half a year. Instead, our partners got this done in weeks, and the FDA approval came within days of submission. It’s unheard of.”
Not only was this the fastest FDA authorization of a project in UAMS’ history, but the program also represents another first in that it enables the treatment statewide. Typically, UAMS research projects are conducted exclusively at UAMS or with a handful of other sites. However, the convalescent plasma program was specifically written so that other interested health care providers in Arkansas may participate by coordinating with UAMS instead of having to go through a separate authorization process with the FDA.
“This is an example of one of the many things related to COVID-19 that have been fast-tracked as the state, nation, and world look for treatments and cures,” said Cam Patterson, M.D., MBA., UAMS chancellor. “There are currently no approved treatments for COVID-19, so there is a sense of urgency as scientists, public health officials, regulatory staff and others – people who typically stay behind-the-scenes – are hard at work giving our front-line health care workers every tool they might be able to use to help as many patients as possible during these unprecedented times.”
UAMS has previously used convalescent plasma on a case-by-case basis, applying through a separate FDA emergency use authorization process for each patient. This program authorization streamlines that process.
The FDA has approved the investigation of convalescent plasma for COVID-19 because, while unproven, it has been used to treat patients with other severe acute respiratory syndrome (SARS) illnesses. There is anecdotal evidence to suggest it might help some people recover from COVID-19. Further studies and larger clinical trials are necessary before it becomes a standard treatment. However, experiences gained during its expanded access treatment use could be a stepping stone toward clinical trials.
According to the Arkansas Department of Health, more than 2,000 people in Arkansas had recovered from COVID-19.
Candidates for convalescent plasma donation must be fully recovered for at least two weeks, have laboratory-confirmed COVID-19, meet blood donor eligibility criteria, and provide informed consent. To read more about the FDA criteria for donation, visit the FDA website or talk to your doctor. The Arkansas Blood Institute, Community Blood Center of the Ozarks and LifeShare are gathering the donations in Arkansas.
Health care providers interested in using COVID-19 convalescent plasma through the expanded access program are asked to contact Ipe at TIpe@uams.edu.
UAMS is the state’s only health sciences university, with colleges of Medicine, Nursing, Pharmacy, Health Professions and Public Health; a graduate school; a hospital; a main campus in Little Rock; a Northwest Arkansas regional campus in Fayetteville; a statewide network of regional campuses; and eight institutes: the Winthrop P. Rockefeller Cancer Institute, Jackson T. Stephens Spine & Neurosciences Institute, Harvey & Bernice Jones Eye Institute, Psychiatric Research Institute, Donald W. Reynolds Institute on Aging, Translational Research Institute, Institute for Digital Health & Innovation and the Institute for Community Health Innovation. UAMS includes UAMS Health, a statewide health system that encompasses all of UAMS’ clinical enterprise. UAMS is the only adult Level 1 trauma center in the state. UAMS has 3,275 students, 890 medical residents and fellows, and five dental residents. It is the state’s largest public employer with more than 12,000 employees, including 1,200 physicians who provide care to patients at UAMS, its regional campuses, Arkansas Children’s, the VA Medical Center and Baptist Health. Visit www.uams.edu or uamshealth.com. Find us on Facebook, X (formerly Twitter), YouTube or Instagram.###