View Larger Image
Winthrop P. Rockefeller Cancer Institute Director Michael Birrer, M.D., Ph.D., checks on the progress of Dwight Hamilton, an advanced stage kidney cancer patient receiving a novel immunotherapy to treat his disease.
Image by Kenneth Childres
Arkansan Sees Advanced Cancer Disappear with Experimental Drug Offered at UAMS Winthrop P. Rockefeller Cancer Institute
| A Little Rock man with resistant kidney cancer who underwent experimental immunotherapy in Phase 1 Clinical Trials at the University of Arkansas for Medical Sciences (UAMS) Winthrop P. Rockefeller Cancer Institute has experienced a complete remission.
Dwight Hamilton, 58, battled stage 4 renal cell carcinoma for four years. His cancer returned multiple times even after receiving all of the standard cancer treatments – surgery, chemotherapy, radiation and other immunotherapies.
Hamilton is cancer-free after receiving a promising new immunotherapy which was tested for the first time in humans exclusively at UAMS in Arkansas and at 45 other hospitals nationwide. Michael Birrer, M.D., Ph.D., director of the Winthrop P. Rockefeller Cancer Institute and UAMS vice chancellor, oversaw the study in the Phase 1 Cancer Clinical Trials Unit at UAMS, the only academic clinical trials unit in Arkansas focused solely on testing new, early phase cancer therapies.
“In the last 15 years, drug development within the field of oncology has exploded,” said Birrer. “These developments continue to expand our ability to deliver more precise, targeted approaches that can potentially lead to improved outcomes and quality of life for some cancer patients.”
Patients with advanced refractory solid tumors were eligible for the trial, entitled “Phase 1 first in human study of leukocyte immunoglobulin-like receptor B2 inhibitor monoclonal antibody JTX-8064, as monotherapy and in combination with a programmed cell death receptor-1 (PD-1) inhibitor.”
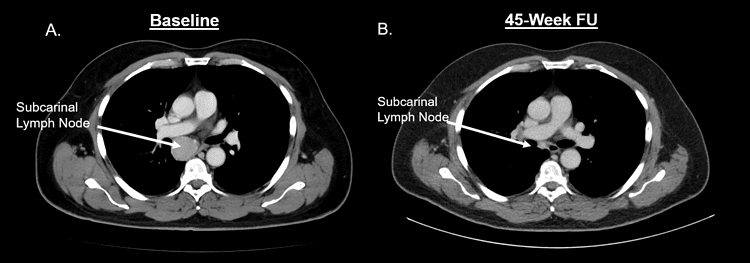
Before and after scans of Dwight’s subcarinal lymph node show the dramatic shrinking of the tumor after 45 weeks.
Hamilton received his first dose of the drug via infusion Feb. 6, 2023. The UAMS team watched with growing excitement as they saw Hamilton’s tumor shrink surprisingly fast. After only three doses, tumors in his chest had shrunk by 33%. After 15 doses given over 11 months, his tumors had completely disappeared.
“In my three decades as an oncologist, his response is unprecedented,” said Birrer. “These and the other new therapies we’re testing have the potential to significantly impact the lives of other patients.”
The trial results are encouraging for the UAMS Cancer Clinical Trials Team that is currently conducting nine Phase 1 clinical trials on new cancer drugs. A total of 140 cancer patients are on interventional treatment trials at UAMS.
Although the immunotherapy trial is now closed, UAMS received permission from the U.S. Food and Drug Administration to let Hamilton stay on the trial drug. He receives the immunotherapy by infusion every few weeks while he is closely monitored. He has had no side effects or complications from the treatment.
“This type of immunotherapy drug turns tumor-growing immune cells into tumor-destroying cells by activating specialized cells known as macrophages,” said Birrer. “Once these cells are activated in this way, the patient’s own immune system adapts and begins to kill cancer cells on its own.”
Immunotherapies are not as widely used as conventional cancer treatments and are often tested as potential cancer treatments as part of early-phase clinical trials. Phase I clinical trials involve small groups of patients to learn more about a drug or treatment’s safety and to identify any potential side effects. In many cases, clinical researchers will also evaluate dosage compared to toxicity. Phase I clinical trials are important to bringing new, innovative approaches to a larger number of cancer patients.
“It’s critical for a cancer center that is worth its salt to have a Phase I unit and a Phase I clinical trial portfolio. This is what our most vulnerable patients need,” Birrer added. “We’re very excited because we have the only academic Phase I unit in the state of Arkansas.”
Hamilton says he feels like a normal person. “I jump for joy right now. I’m thinking about going and dancing and having fun.”