UAMS First in Nation to Offer Groundbreaking Therapy for Treatment-Resistant Depression
| LITTLE ROCK — The University of Arkansas for Medical Sciences (UAMS) is the first medical facility in the United States to provide an innovative therapeutic treatment for major depressive disorder (MDD).
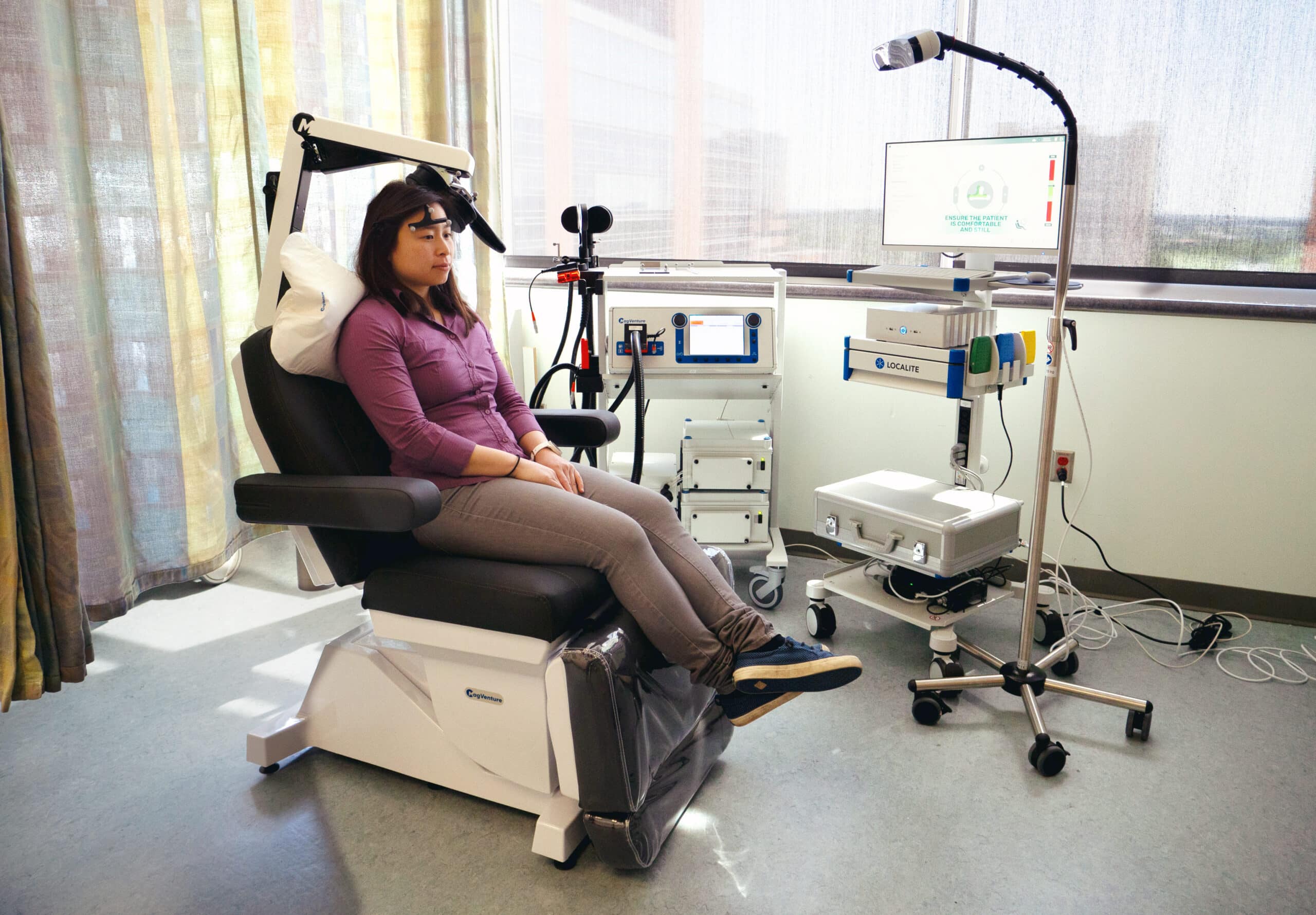
The SAINT® neuromodulation system, developed by Magnus Medical, Inc., has been approved by the U.S. Food and Drug Administration to treat adults with depression who have not achieved improvement in their condition from the use of antidepressant medications. SAINT works by leveraging structural and functional magnetic resonance imaging (MRI) scans to inform a proprietary algorithm that pinpoints the optimal anatomical target for precise neurostimulation in individuals with major depression.
The treatment is performed on an accelerated, five-day timeline, reducing the patient’s treatment time from weeks to days. In previous clinical trials, treatment with SAINT for MDD resulted in a significant reduction in depressive symptoms at four weeks post-treatment following the five-day treatment protocol. Currently, SAINT can only be provided to patients who are being treated as inpatients.
“We are thrilled to be the first site in the nation to offer the breakthrough SAINT neuromodulation system for individuals suffering from depression, and I am very optimistic that this new approach will change people’s lives,” said Laura B. Dunn, M.D., chair of the Department of Psychiatry and director of the Psychiatric Research Institute at UAMS.
“With our interventional psychiatry program, the Helen L. Porter and James T. Dyke Brain Imaging Research Center, and our inpatient and outpatient treatment programs all housed under one roof, our teams of mental health experts will work seamlessly together to implement the SAINT protocol, tailored to each patient’s individual needs,” she added. “We are excited to bring the future of brain science directly to the people of Arkansas and the entire region.”
Patients in the SAINT treatment protocol undergo an MRI scan in the Brain Imaging Research Center, which takes approximately 45 minutes, to pinpoint the optimal anatomical target for precise transcranial magnetic stimulation (TMS). The TMS treatment consists of 10 sessions per day for five consecutive days. Each session includes 10 minutes of stimulation followed by a 50-minute rest period.
“The innovative SAINT protocol provides neuroscience-based, targeted, personalized, noninvasive rapid treatment for patients with one of the most disabling psychiatric conditions — major depression. At the Psychiatric Research Institute, our mission is to provide outstanding, expert-driven psychiatric care for our patients,” said Dunn.
In addition to the new SAINT neuromodulation system, PRI’s Interventional Psychiatry program offers several treatment options for individuals whose depression has not responded to traditional medication- and psychotherapy-based treatments. These treatments include Spravato (intranasal ketamine), intravenous ketamine, electroconvulsive therapy (ECT), and now accelerated transcranial magnetic stimulation using the SAINT neuromodulation system.
“Because each individual’s needs are different, new patients will be seen for a consultation appointment with the Interventional Psychiatry program at PRI,” said Amy Grooms, M.D., a psychiatrist with the Interventional Psychiatry program. “During that appointment, the patient will receive an in-depth psychiatric evaluation with one of our interventional psychiatrists, who specialize in the care of patients suffering from treatment-resistant depression.”
Individuals who are interested in learning more or who would like to schedule a consultation to evaluate their treatment options, should contact the Interventional Psychiatry Program at PRI, by visiting their website at: psychiatry.uams.edu/clinical-care/interventional-psychiatry/ or by calling (501) 526-8650.